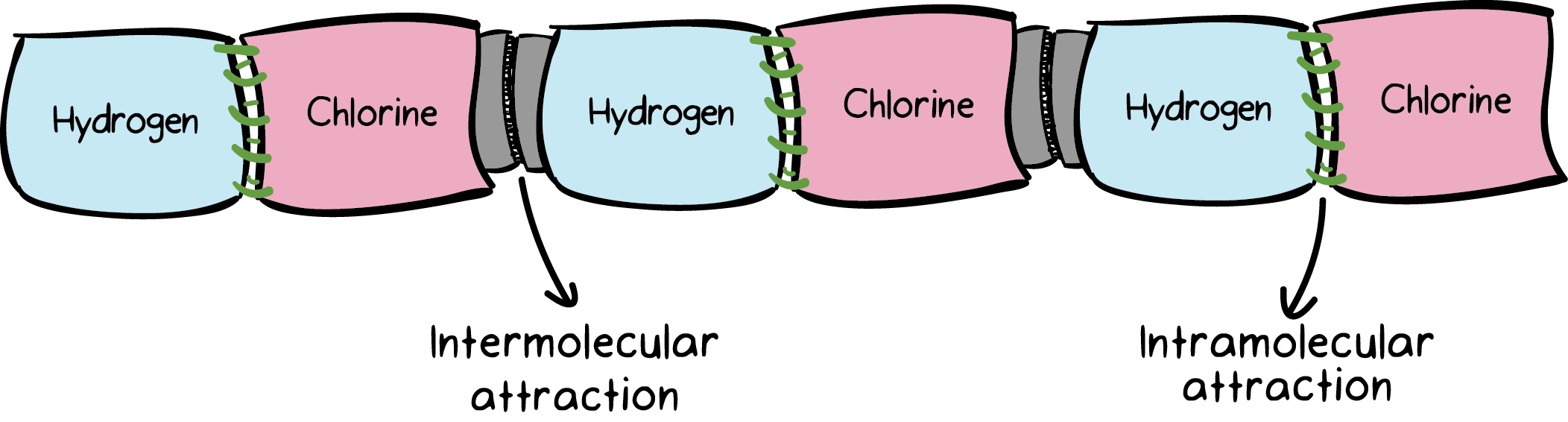
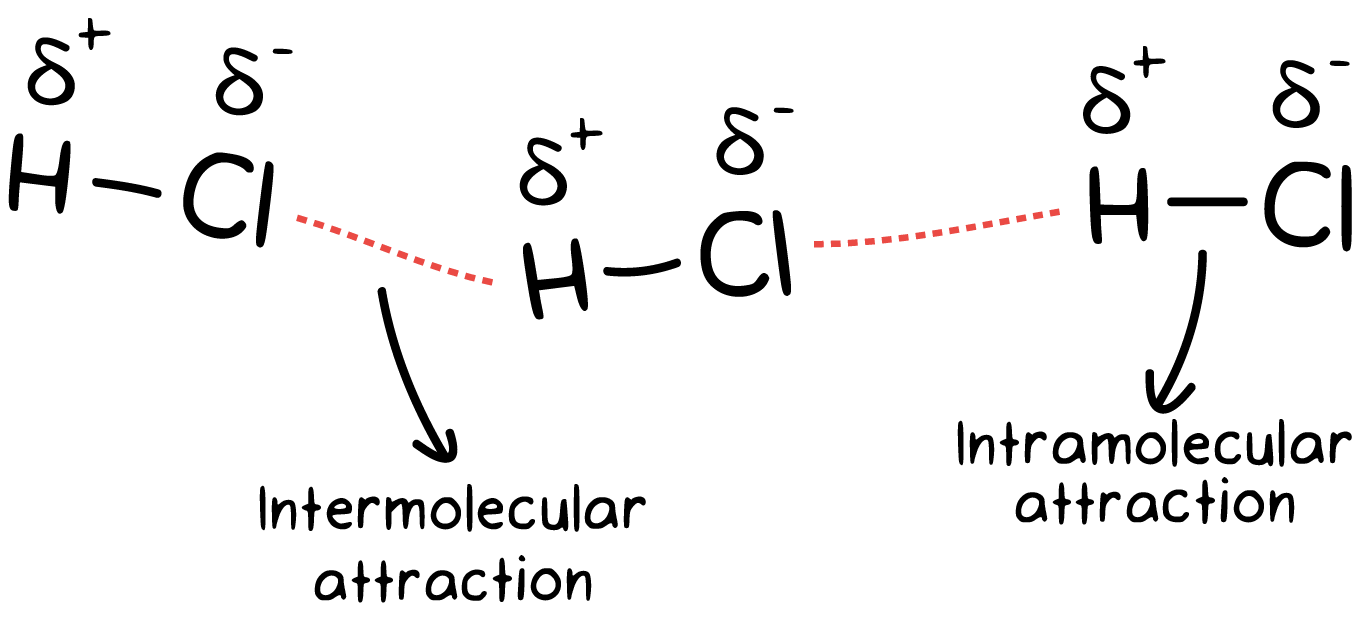
IDEAL GAS
|
REAL GAS
|
PV=nRT
|
(P + an2)(V
- nb)=nRT
V2
|
Condition
: Low Pressure, High temperature
|
Condition:
Low temperature, high Pressure
|
When pressure
decrease, Volume increase
·
Distance between gas molecules far apart
·
The volume of gas too small compared to volume of the
container
·
Thus volume of gas molecules is negligible
|
When
pressure increase, volume decrease
·
The distance between gas molecules become closer
·
Volume of gas become significant and cannot be neglect
|
When temperature
increase
·
Kinetic energy of gas molecules increase
·
Molecules move faster and able to overcome the
intermolecular forces between molecules
· Thus, the attractive forces between gas molecules can
be neglect.
|
When temperature
decrease
·
Kinetic energy of gas decrease
·
Gas molecules are close to each other and move slower
·
The attractive forces between gas molecules become
significant
|
In Van Der Waals equation:6
n term of pressure (P
V2
|
In term of volume
|
At low
temperature, average kinetic energy of gas decrease
|
At
high pressure, volume of container decrease
|
Gas move
slower and intermolecular forces become significant
|
Gas molecules
are much closer and volume of gas molecules become significant
|
The frequency
of collision on the wall container decrease
|
|
As a
result:
P real< P ideal
|
As a
result:
Vreal > V ideal
|
The factor
an2/V2 is
added to the P term correct the
pressure of real gas which is smaller than ideal gas
|
The factor
nb is substracted from the V term to correct the volume of real
gas which is larger than ideal gas.
|
a is constant to correct for intermolecular
forces
|
b is constant to correct for volume occupied
by gas molecules
|
The higher
the a value, the stronger the
attractive forces between gas molecules
|
The higher
the b value, the bigger the volume
occupied by gas molecules
|