Intramolecular and intermolecular forces
Analogy to understand about intramolecular forces and intermolecular forces.
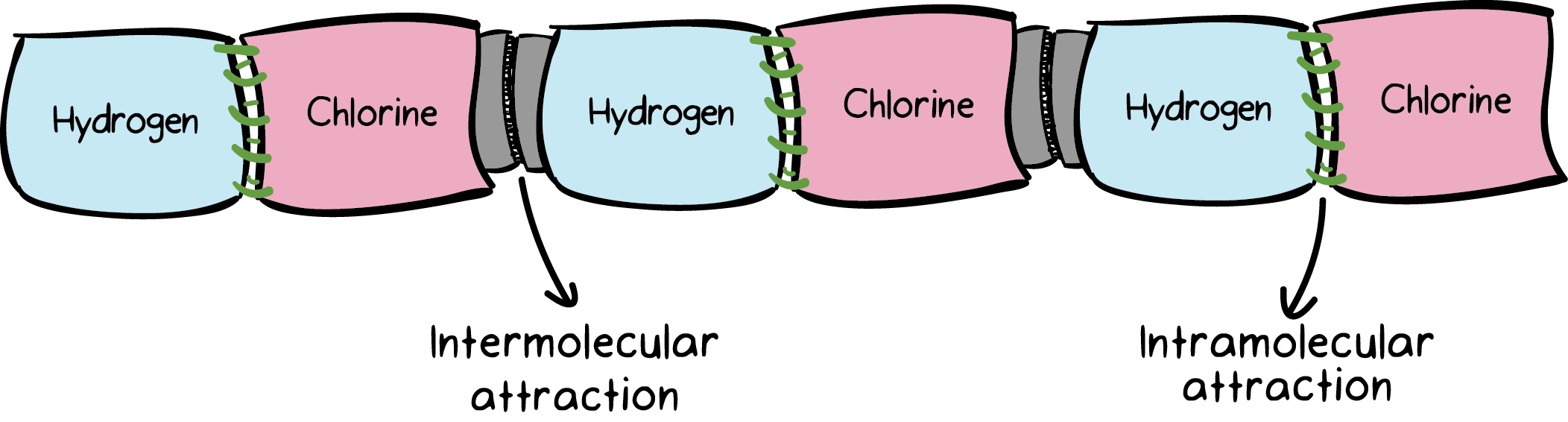 |
We have six towels—three are purple in color, labeled hydrogen and three are pink in color, labeled chlorine. We are given a sewing needle and black thread to sew one hydrogen towel to one chlorine towel. After sewing, we now have three pairs of towels: hydrogen sewed to chlorine. The next step is to attach these three pairs of towels to each other. For this we use Velcro (grey colour as label intermolecular attraction) as shown above .
So, the result of this exercise is that we have six towels attached to each other through thread and Velcro. Now if I ask you to pull this assembly from both ends, what do you think will happen? The Velcro junctions will fall apart while the sewed junctions will stay as is. The attachment created by Velcro is much weaker than the attachment created by the thread that we used to sew the pairs of towels together. A slight force applied to either end of the towels can easily bring apart the Velcro junctions without tearing apart the sewed junctions.
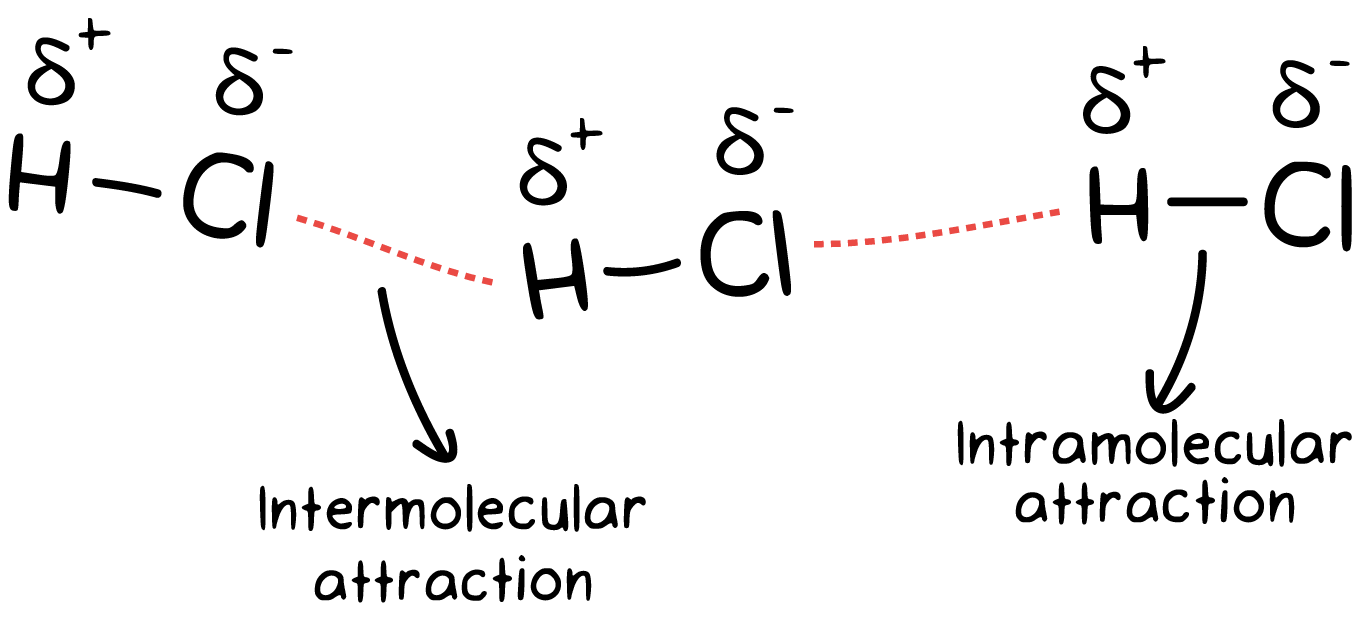
Exactly the same situation exists in molecules. Just imagine the towels to be real atoms, such as hydrogen and chlorine. These two atoms are bound to each other through a polar covalent bond—analogous to the thread. Each hydrogen chloride molecule in turn is bonded to the neighboring hydrogen chloride molecule through a dipole-dipole attraction—analogous to Velcro. We’ll talk about dipole-dipole interactions in detail a bit later. The polar covalent bond is much stronger in strength than the dipole-dipole interaction. The former is termed an intramolecular attraction while the latter is termed an intermolecular attraction.
So now we can define the two forces:
Intramolecular forces are the forces that hold atoms together within a molecule. Intermolecular forces are forces that exist between molecules.



No comments:
Post a Comment